Articles
Improve energy consumption through better electrodes
Electrolyzer Technologies (Column)
W. THORNHILL, Jolt Solutions, Barcelona, Spain
Green hydrogen (H2) production is dominated by alkaline water electrolyzers (~80%), with other technologies such as proton exchange membrane (PEM) electrolyzers (~20%), emerging anion exchange membrane (AEM) electrolyzers and solid oxide electrolyzer cell (SOEC) technologies comprising a smaller percentage of deployed capacity. Alkaline water electrolysis (AWE), with its proven track record, lower-cost equipment and scalability, is often the go-to choice for large-scale H2 projects [> 100 megawatt (MW)]. AWE relies on activated electrodes—typically nickel-based electrodes activated with catalysts—to perform water splitting and gas formation. The selection of the electrode package can have a big impact on the performance of the electrolyzer system.
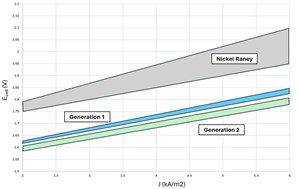
Traditionally, AWE systems have used a combination of nickel anodes and nickel Raney cathodes. The anode, typically not activated, has historically suffered from oxidation as the equipment cycles through voltage steps, resulting in a need to maintain a high baseload with limited shutdowns. This has also reduced the current density at which the equipment can operate, although lower current densities do lead to higher gas production efficiency (FIG. 1).
Nickel Raney, a high-surface-area nickel coating deposited on the surface of an underlying nickel substrate, offers good levels of performance on the cathode but can be prone to early degradation. In some instances, nickel Raney may also be used on both the anode and cathode.
Most alkaline electrolyzers operate in the low current density realm (0.25A/cm2–0.5A/cm2), but there are exceptions. Some electrolyzer companies design equipment to operate at higher current densities in excess of 1A/cm2. Typically, this type of equipment uses a precious metal catalyst to achieve higher performance and must be iron-free, which can increase the cost of the stack.
When it comes to field deployment, the initial cost of the electrolyzer (including the stack) is a major capital expenditure (CAPEX) concern. However, the greatest impact on the levelized cost of H2 (LCOH) is the cost and amount of renewable electricity used in H2 production. The stack design and the components used in the stack have a big impact on this electrical efficiency. H2 project managers are often willing to pay more for original equipment if it uses less electricity per kilogram (kg) of H2 and maintains that efficiency longer (through higher-durability components, resulting in lower degradation and longer intervals between services). There is also an increasing demand for equipment with more flexibility to accommodate variable current from renewable energy sources.
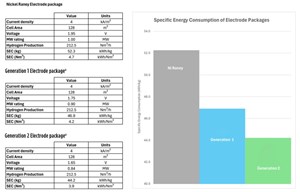
FIG. 2 shows the impact of using more advanced electrodes on the specific energy consumption in kilowatt hours (kWh)/kg and kWh/normal cubic meters (Nm3). These values only consider the balance of stack (BoS), and it should be noted that there will be an effect from the balance of plant (BoP). Even so, changing the electrodes to next-generation designs can significantly reduce the energy consumption.
Newer coatings can also improve durability, even against repeat shutdown cycles as may be experienced when coupling electrolysis with renewable energy. Traditional electrodes are normally based on nickel-woven or expanded mesh. This is largely because of the limitations of older coating techniques such as electroplating, electrodeposition or thermal decomposition.
Modern coating techniquesa can coat much more complex electrodes based on nickel substrates, such as foam and felt. These higher-surface-area base materials, in conjunction with new generations of catalysts, lead to a combined effect in terms of performance advantages. The increased surface area provides an initial performance boost, and the greater catalyst deposition creates a compounded effect.
There are several new coating techniques being explored but given the scale of production demanded by gigawatt scale projects, there should be serious considerations to the cost and speed of the production. High-end, extremely high-performing electrodes can be created on a small scale by using exotic or complicated materials, or high energy-intensive or slow processes but often these cannot realistically be deployed at scale (FIG. 3).
In some cases, new coating techniquesa can scale faster and at lower cost than traditional methods, meaning they can support the gigawatt ambitions of many companies. Another key factor to increasing electrolyzer efficiency is reducing the downtime during service intervals. When possible, electrode production should occur as close to the deployed electrolyzer as possible to ensure short maintenance periods. If such a service was available, more frequent service intervals could be employed, ensuring the electrolyzer runs efficiently. Strategically distributed, small-scale modular electrode coating lines will be essential in the future. H2T
NOTE
a Jolt’s Sparkfuze process
About the author

WAYNE THORNHILL is the Global Sales Director, Electrode Solutions for Jolt Solutions. The author can be reached at w.thornhill@jolt-solutions.com